Review Article |
|
Corresponding author: Mohd Alaraj ( ibrahim_naseem@yahoo.com ) Academic editor: Georgi Momekov
© 2022 Mohd Alaraj, Fahaad S. Alenazi, Dania Hassan, Ashfaque Hossain.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Alaraj M, Alenazi FS, Hassan D, Hossain A (2022) Vitamin D as a drug: new therapeutic approaches. Pharmacia 69(3): 765-770. https://doi.org/10.3897/pharmacia.69.e85057
|
Abstract
Vitamin D is one of the essential vitamins and has recently been demonstrated to be much more important for the appropriate functioning of the human body and well-being than initially believed. Although vitamin D is mainly known for its link with bone fractures and bone diseases, recent studies revealed that vitamin D and its analogues have revealed many pharmacological actions covering the regulation of cell growth, inhibition of inflammation, and improvement of neuromuscular function and immune function. Moreover, vitamin D and its analogues are reported to have role in different types of cancers, skin diseases, diabetes mellitus and infections caused by different bacterial and viral pathogens including SARS-CoV-2. The goal of this study is to evaluate the scientific literature on therapeutic uses of vitamin D and its analogues against different diseases and health condition. Special attention has been given to COVID-19 infection, cancer, skin diseases, and diabetes. The molecular mechanisms involved are also explored.
Keywords
calcidiol, calcitriol, vitamin D3, SARS-CoV-2
Introduction
Vitamin D comprises a collection of fat-soluble secosteroid organic compounds that have anti-rickets properties. The key D vitamins are vitamin D2 (ergocalciferol) and D3 (cholecalciferol) (
Occurrence
It is estimated that 90–95% of the vitamin D required is generated through skin synthesis, while diet is the secondary source of this vitamin in the human body (
Several years ago, it was found that actual vitamin D3 is calcitriol, while cholecalciferol is a provitamin that is converted to calcitriol in the kidneys (Whiting et al. 2021). Alfacalcide is derived from vitamin D3 as a very strong metabolite that is used in conditions when the body is unable to convert cholecalciferol into calcifediol due to kidney dysfunction. Thus, it is used for patients with liver and kidney injury who have symptoms of hypovitaminosis D (
Pharmacokinetics
Absorption; vitamin D3 is a lipid-soluble molecule that is absorbed into the lacteals in the gastrointestinal tract through chylomicrons. It is then transported via the lymphatic system and subsequently into the blood stream (
Pharmacodynamics
The role of the vitamin D receptor in the functioning of vitamin D3 in the body
Vitamin D receptor (VDR) mediates the hormonal function of calcitriol in several physiological processes in the human body (Uberti 2016;
Calcitriol interacts with the receptors via two pathways: the classical genomic associated pathway, where calcitriol interacts with the nuclear VDR, and the non-genomic pathway, where this vitamin interacts with the membrane VDR (
Role in the body
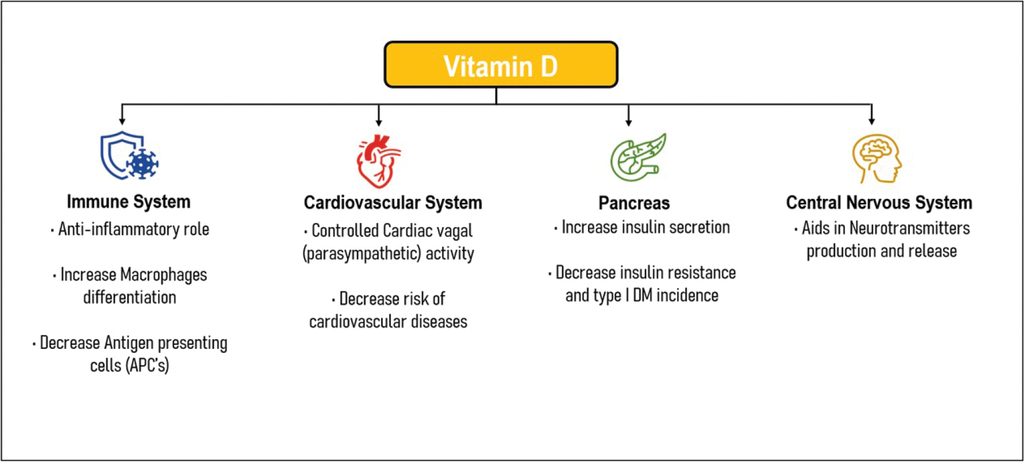
Vitamin D is vital for the appropriate functioning of the whole body, particularly through its effects on metabolic processes (
Potential therapeutic uses
SARS-CoV-2 virus infection
Vitamin D3 has been suggested as a possible adjunct therapy for COVID-19 due to the in vitro observations of antiviral and protective effects against respiratory tract infections (including rhinovirus and respiratory syncytial virus infections), as well as its immunomodulation effects (
A number of clinical studies have examined or are currently exploring the relationship of vitamin D3 with SARS-CoV-2 infection. A retrospective cohort study showed that people infected with the SARS-CoV-2 virus had more than two-times lower serum concentrations of calcidiol on average than those in the control group (11.1 ng/ml and 24.6 ng/ml, respectively, P = 0.004) (
Vitamin D is reported to have a protective effect against SARS-CoV-2 infection, which prompted its inclusion in both preventive and treatment regimens for COVID-19. Serum vitamin D concentration is reported to serve as predictor of SARS-CoV-2 infection, which is independent of age, sex, race and attitude (
Although some progresses have been made in understanding the molecular basis of action of vitamin D, the total picture is not clear. However, enhanced expression of antimicrobial proteins, defensin and cathelicidin which are reported to play important role in the infection outcome process of various bacterial and viral agent(
SARS-CoV-2 infection is often leads to neurological symptoms such as loss of smell and taste, dizziness and confusion as a result of damages to the neurons caused by the virus. Vitamin D exerts neuroprotective effect by regulating the production of neurophilins, which are key factors modulating survival, differentiation and proliferation of neurons. Studies have shown that vitamin D promotes the migration and proliferation of oligodendrocytes, enhancing the remyelination of damaged neurons in animal models (
Cancer diseases
Increasing evidence suggests that vitamin D and its derivatives exhibit antitumor effects in mice (
Nonetheless, the key adverse effects of vitamin D – hypercalcemia and hypercalciuria – restrict its utilization in cancer therapy (
Skin diseases
Vitamin D can increase the production of several antimicrobial peptides as well as the effects of cytokine and T helper type 2 cells. Thus, using this vitamin could decrease the risk of skin infection (
Although the mechanism of action of vitamin D and its analogues is not fully understood, it is known that this vitamin can augment keratinocyte differentiation and either activate or inhibit keratinocyte growth depending on the dose (
Diabetes mellitus
Both type 1 and type 2 diabetes can be prevented or treated with vitamin D and its analogues. This results from normalizing the function of the immune system, promoting B-cell survival and function, facilitating insulin secretion and glucose uptake, and controlling insulin-receptor gene expression (Sintov et al. 2014;
Conclusion
A growing body of clinical, experimental, and epidemiological studies suggests that vitamin D and its analogues may have therapeutic potential, particularly for the prevention and treatment of COVID-19 infection, various cancers, diabetes, and some skin diseases. Nevertheless, further long-term randomized controlled trials on vitamin D and its analogues are required to better understand their efficiency in the prevention and therapy for diseases, their adverse effects, and their molecular mechanisms of actions.
Acknowledgements
The authors are grateful to the Middle East University, Amman, Jordan, for the financial support to cover the publication fee of this research article.
References
- Abdel-Rehim WM, El-Tahan RA, El-Tarawy MA, Shehata RR, Kamel MA (2019) The possible antidiabetic effects of vitamin D receptors agonist in rat model of type 2 diabetes. Molecular and cellular biochemistry 450(1): 105–112. https://doi.org/10.1007/s11010-018-3377-x
- Ali N (2020) Role of vitamin D in preventing of COVID-19 infection, progression and severity. Journal of infection and public health 13(10): 1373–1380. https://doi.org/10.1016/j.jiph.2020.06.021
- Annweiler C, Beaudenon M, Gautier J, Simon R, Dubée V, Gonsard J, Parot-Schinkel E (2020) COvid-19 and high-dose VITamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): study protocol for a randomized controlled trial. Trials 21(1): 1–10. https://doi.org/10.1186/s13063-020-04928-5
- Aslam A, Ahmad J, Baghdadi MA, Idris S, Almaimani R, Alsaegh A, Alhadrami M, Refaat B (2021) Chemopreventive effects of vitamin D3 and its analogue, paricalcitol, in combination with 5-fluorouracil against colorectal cancer: The role of calcium signalling molecules. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1867(3): е166040. https://doi.org/10.1016/j.bbadis.2020.166040
- Aydın CG, Dinçel YM, Arıkan Y, Taş SK, Deniz S (2019) The effects of indoor and outdoor sports participation and seasonal changes on vitamin D levels in athletes. SAGE Open Medicine 7: 2050312119837480. https://doi.org/10.1177/2050312119837480
- Bakke D, Sun J (2018) Ancient nuclear receptor VDR with new functions: microbiome and inflammation. Inflammatory bowel diseases 24(6): 1149–1154. https://doi.org/10.1093/ibd/izy092
- Ben-Shabat S, Benisty R, Wormser U, Sintov AC (2005) Vitamin D3–based conjugates for topical treatment of psoriasis: synthesis, antiproliferative activity, and cutaneous penetration studies. Pharmaceutical research 22(1): 50–57. https://doi.org/10.1007/s11095-004-9008-0
- Bouillon R (2017) Comparative analysis of nutritional guidelines for vitamin D. Nature Reviews Endocrinology 13(8): 466–479. https://doi.org/10.1038/nrendo.2017.31
- Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, Lips P, Munns CF, Lazaretti-Castro M, Giustina A, Bilezikian J (2019) Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocrine reviews 40(4): 1109–1151. https://doi.org/10.1210/er.2018-00126
- Charoenngam N, Kalajian TA, Shirvani A, Yoon GH, Desai S, McCarthy A, Apovian CM, Holick MF (2021) A pilot-randomized, double-blind crossover trial to evaluate the pharmacokinetics of orally administered 25-hydroxyvitamin D3 and vitamin D3 in healthy adults with differing BMI and in adults with intestinal malabsorption. The American journal of clinical nutrition 114(3): 1189–1199. https://doi.org/10.1093/ajcn/nqab123
- Chen PZ, Li M, Duan XH, Jia JY, Li JQ, Chu RA, Yu C, Han JH, Wang H (2016) Pharmacokinetics and effects of demographic factors on blood 25 (OH) D3 levels after a single orally administered high dose of vitamin D3. Acta Pharmacologica Sinica 37(11): 1509–1515. https://doi.org/10.1038/aps.2016.82
- Chen Y, Hou J, Xiao Z, Zhao Y, Du F, Wu X, Li M, Zhang L, Cho CH, Wen Q, Hu W (2021) The Role of Vitamin D in Gastrointestinal Diseases: Inflammation, Gastric Cancer, and Colorectal Cancer. Current medicinal chemistry 29(22): 3836–3856. https://doi.org/10.2174/0929867328666211111163304
- D’Avolio A, Avataneo V, Manca A, Cusato J, De Nicolò A, Lucchini R, Keller F, Cantù M (2020) 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients 12(5): 1359. https://doi.org/10.3390/nu12051359
- Fiske CT, Blackman A, Maruri F, Rebeiro PF, Huaman M, Kator J, Scott Algood HM, Sterling TR (2019) Increased Vitamin D Receptor Expression from Macrophages after Stimulation with M. tuberculosis Among Persons Who Have Recovered from Extrapulmonary Tuberculosis. BMC Infectious Diseases 19: e366. https://doi.org/10.1186/s12879-019-3958-7
- Fujita H, Asahina A, Komine M, Tamaki K (2007) The direct action of 1α, 25 (OH) 2-vitamin D3 on purified mouse Langerhans cells. Cellular immunology 245(2): 70–79. https://doi.org/10.1016/j.cellimm.2007.03.007
- Galassi A, Ciceri P, Porata G, Iatrino R, Boni Brivio G, Fasulo E, Magagnoli L, Stucchi A, Frittoli M, Cara A, Cozzolino M (2021) Current treatment options for secondary hyperparathyroidism in patients with stage 3 to 4 chronic kidney disease and vitamin D deficiency. Expert Opinion on Drug Safety 20(11): 1333–1349. https://doi.org/10.1080/14740338.2021.1931117
- Geilen CC, Bektas M, Wieder T, Kodelja V, Goerdt S, Orfanos CE (1997) 1α, 25-Dihydroxyvitamin D3 induces sphingomyelin hydrolysis in HaCaT cells via tumor necrosis factor α. Journal of Biological Chemistry 272(14): 8997–9001. https://doi.org/10.1074/jbc.272.14.8997
- Gomez-Pinedo U, Cuevas JA, Benito-Martín MS, Moreno-Jiménez L, Esteban-Garcia N, Torre-Fuentes L, Matias-Guiu JA, Pytel V, Montero P, Matias-Guiu J (2020) Vitamin D Increases Remyelination by Promoting Oligodendrocyte Lineage Differentiation. Brain and Behavior 10(1): e01498. https://doi.org/10.1002/brb3.1498
- Guo C, Rosoha E, Lowry MB, Borregaard N, Gombart AF (2013) Curcumin induces human cathelicidin antimicrobial peptide gene expression through a vitamin D receptor-independent pathway. The Journal of Nutritional Biochemistry 24(5): 754–759. https://doi.org/10.1016/j.jnutbio.2012.04.002
- Gu J, Rodriguez K, Yang S, Ociepa M, Wilke H, Abrishami A, Joergensen L, Skak-Nielsen T, Chen J (2021) Convergent Total Synthesis of (+)-Calcipotriol: A Scalable, Modular Approach to Vitamin D Analogs. ChemRxiv. Cambridge: Cambridge Open Engage.
- Haddad JG, Matsuoka LY, Hollis BW, Hu YZ, Wortsman J (1993) Human plasma transport of vitamin D after its endogenous synthesis. The Journal of Clinical Investigation 91(6): 2552–2555. https://doi.org/10.1172/JCI116492.
- Hansdottir S, Monick MM (2011) Vitamin D Effects on Lung Immunity and Respiratory Diseases. Vitamins and Hormones 86: 217–237. 10.1016/B978-0-12-386960-9.00009-5
- Hassan AN, Toma T, Ciftci H, Biswas T, Tahara Y, Radwan MO, Tateishi H, Fujita M, Otsuka M (2022) A vitamin D C/D ring-derived compound with cytotoxicity. Medicinal Chemistry Research 31: 1120–1125. https://doi.org/10.1007/s00044-021-02842-2
- Hnokaew P, Yammuen-Art SYA (2021) Vitamin D2 production and in vitro ruminal degradation of UV-B irradiated vitamin D enriched yeast in Thai native cattle. Veterinary Integrative Sciences 19(3): 537–556. https://doi.org/10.12982/VIS.2021.042
- Holick MF (2007) Vitamin D deficiency. New England journal of medicine 357(3): 266–281. https://doi.org/10.1056/NEJMra070553
- Ilie PC, Stefanescu S, Smith L (2020) The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging clinical and experimental research 32(7): 1195–1198. https://doi.org/10.1007/s40520-020-01570-8
- Jaun F, Boesing M, Lüthi-Corridori G, Abig K, Makhdoomi A, Bloch N, Lins C, Raess A, Grillmayr V, Haas P, Schuetz P, Gabutti L, Muser J, Leuppi-Taegtmeyer AB, Giezendanner S, Brändle M, Leuppi JD (2022) High-dose vitamin D substitution in patients with COVID-19: study protocol for a randomized, double-blind, placebo-controlled, multi-center study – VitCov Trial. Trials 23(1): 1–11. https://doi.org/10.1186/s13063-022-06016-2
- Kearns MD, Alvarez JA, Seidel N, Tangpricha V (2015) Impact of vitamin D on infectious disease. The American journal of the medical sciences 349(3): 245–262. https://doi.org/10.1097/MAJ.0000000000000360
- Kechichian E, Ezzedine K (2018) Vitamin D and the skin: an update for dermatologists. American journal of clinical dermatology 19(2): 223–235. https://doi.org/10.1007/s40257-017-0323-8
- Kim SH, Chen G, King AN, Jeon CK, Christensen PJ, Zhao L, Simpson RU, Thomas DG, Giordano TJ, Brenner DE, Hollis B (2012) Characterization of vitamin D receptor (VDR) in lung adenocarcinoma. Lung cancer 77(2): 265–271. https://doi.org/10.1016/j.lungcan.2012.04.010
- Lee SM, Meyer MB, Benkusky NA, O’Brien CA, Pike JW (2018) The impact of VDR expression and regulation in vivo. The Journal of steroid biochemistry and molecular biology 177: 36–45. https://doi.org/10.1016/j.jsbmb.2017.06.002
- Lespessailles E, Toumi H (2017) Vitamin D alteration associated with obesity and bariatric surgery. Experimental Biology and Medicine 242(10): 1086–1094. https://doi.org/10.1177/1535370216688567
- Li J, Luco AL, Camirand A, St-Arnaud R, Kremer R (2021) Vitamin D regulates CXCL12/CXCR4 and epithelial-to-mesenchymal transition in a model of breast cancer metastasis to lung. Endocrinology 162(7): bqab049. https://doi.org/10.1210/endocr/bqab049
- Ling SF, Broad E, Murphy R, Pappachan JM, Pardesi-Newton S, Kong MF, Jude EB (2020) High-dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID-19: a cross-sectional multi-centre observational study. Nutrients 12(12): 3799. https://doi.org/10.3390/nu12123799
- McConnell DD, McGreevy JW, Williams MN, Litofsky NS (2018) Do anti-oxidants vitamin D3, melatonin, and alpha-lipoic acid have synergistic effects with temozolomide on cultured glioblastoma cells? Medicines 5(2): 58. https://doi.org/10.3390/medicines5020058
- McCormack PL (2011) Spotlight on calcipotriene/betamethasone dipropionate in psoriasis vulgaris of the trunk, limbs, and scalp. American journal of clinical dermatology 12(6): 421–424. https://doi.org/10.2165/11207670-000000000-00000
- Melguizo-Rodríguez L, Costela-Ruiz VJ, García-Recio E, Luna-Bertos D, Ruiz C, Illescas-Montes R (2021) Role of Vitamin d in the Metabolic Syndrome. Nutrients 13(3): 830. https://doi.org/10.3390/nu13030830
- Melguizo-Rodríguez L, Costela-Ruiz VJ, García-Recio E, Luna-Bertos D, Ruiz C, Illescas-Montes R (2020) Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA network open 13(9): e2019722. https://doi.org/10.3390/nu13030830
- Prentice RL, Pettinger MB, Jackson RD, Wactawski-Wende J, Lacroix AZ, Anderson GL, Chlebowski RT, Manson JE, Van Horn L, Vitolins MZ, Datta M (2013) Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporosis International 24(2): 567–580. https://doi.org/10.1007/s00198-012-2224-2
- Raposo L, Martins S, Ferreira D, Guimarães JT, Santos AC (2017) Vitamin D, parathyroid hormone and metabolic syndrome-the PORMETS study. BMC endocrine disorders 17(1): 1–10. https://doi.org/10.1186/s12902-017-0221-3
- Rizzoli R (2021) Vitamin D supplementation: upper limit for safety revisited?. Aging clinical and experimental research 33(1): 19–24. https://doi.org/10.1007/s40520-020-01678-x
- Saksa N, Neme A, Ryynänen J, Uusitupa M, de Mello VD, Voutilainen S, Nurmi T, Virtanen JK, Tuomainen TP, Carlberg C (2015) Dissecting high from low responders in a vitamin D3 intervention study. The Journal of Steroid Biochemistry and Molecular Biology 148: 275–282. https://doi.org/10.1016/j.jsbmb.2014.11.012
- Schneider J, Jeon YW, Suh YJ, Lim ST (2022) Effects of Ruxolitinib and Calcitriol Combination Treatment on Various Molecular Subtypes of Breast Cancer. International Journal of Molecular Sciences 23(5): 2535. https://doi.org/10.3390/ijms23052535
- Shang M, Sun J (2017) Vitamin D/VDR, probiotics, and gastrointestinal diseases. Current medicinal chemistry 24(9): 876–887. https://doi.org/10.2174/0929867323666161202150008
- Sulli A, Gotelli E, Casabella A, Paolino S, Pizzorni C, Alessandri E, Grosso M, Ferone D, Smith V, Cutolo M (2021) Vitamin D and lung outcomes in elderly COVID-19 patients. Nutrients 13(3): 717. https://doi.org/10.1016/j.drudis.2014.06.008
- Tan CW, Ho LP, Kalimuddin S, Cherng BP, Teh YE, Thien SY, Wong HM, Tern PJ, Chandran M, Chay JW, Nagarajan C (2020) Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrition 79: е111017. https://doi.org/10.1016/j.nut.2020.111017
- Tomaszewska A, Rustecka A, Lipińska-Opałka A, Piprek RP, Kloc M, Kalicki B, Kubiak JZ (2022) The Role of Vitamin D in COVID-19 and the Impact of Pandemic Restrictions on Vitamin D Blood Content. Frontiers in pharmacology 13: е836738. https://doi.org/10.3389/fphar.2022.836738
- Uberti F, Bardelli C, Morsanuto V, Ghirlanda S, Molinari C (2016) Role of vitamin D 3 combined to alginates in preventing acid and oxidative injury in cultured gastric epithelial cells. BMC gastroenterology, 16(1): 1–13. https://doi.org/10.1186/s12876-016-0543-z
- Umar M, Sastry KS, Al Ali F, Al-Khulaifi M, Wang E, Chouchane AI (2018) Vitamin D and the pathophysiology of inflammatory skin diseases. Skin pharmacology and physiology 31(2): 74–86. https://doi.org/10.1159/000485132
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD (2001) The sequence of the human genome. science 291(5507): 1304–1351. https://doi.org/10.1126/science.1058040
- Wang R, DeGruttola V, Lei Q, Mayer KH, Redline S, Hazra A, Mora S, Willett WC, Ganmaa D, Manson JE (2021a) The vitamin D for COVID-19 (VIVID) trial: A pragmatic cluster-randomized design. Contemporary clinical trials 100: е106176. https://doi.org/10.1016/j.cct.2020.106176
- Wang W, Hu W, Xue S, Chen Q, Jiang Y, Zhang H, Zuo W (2021b) Vitamin D and Lung Cancer; Association, Prevention, and Treatment. Nutrition and Cancer 73(11–12): 2188–2200. https://doi.org/10.1080/01635581.2020.1844245
- Whiting SJ, Calvo MS (2021) Vitamin D: Nutrition Information Brief. Advances in Nutrition 12(5): 2037–2039. https://doi.org/10.1093/advances/nmab051
- Wisniewska A, Szypowska A (2021) The role of vitamin D in selected autoimmune diseases. Roczniki Państwowego Zakładu Higieny 72(2): 111–121. https://doi.org/10.32394/rpzh.2021.0156
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF (2000) Decreased bioavailability of vitamin D in obesity. The American journal of clinical nutrition 72(3): 690–693. https://doi.org/10.1093/ajcn/72.3.690