|
||
|
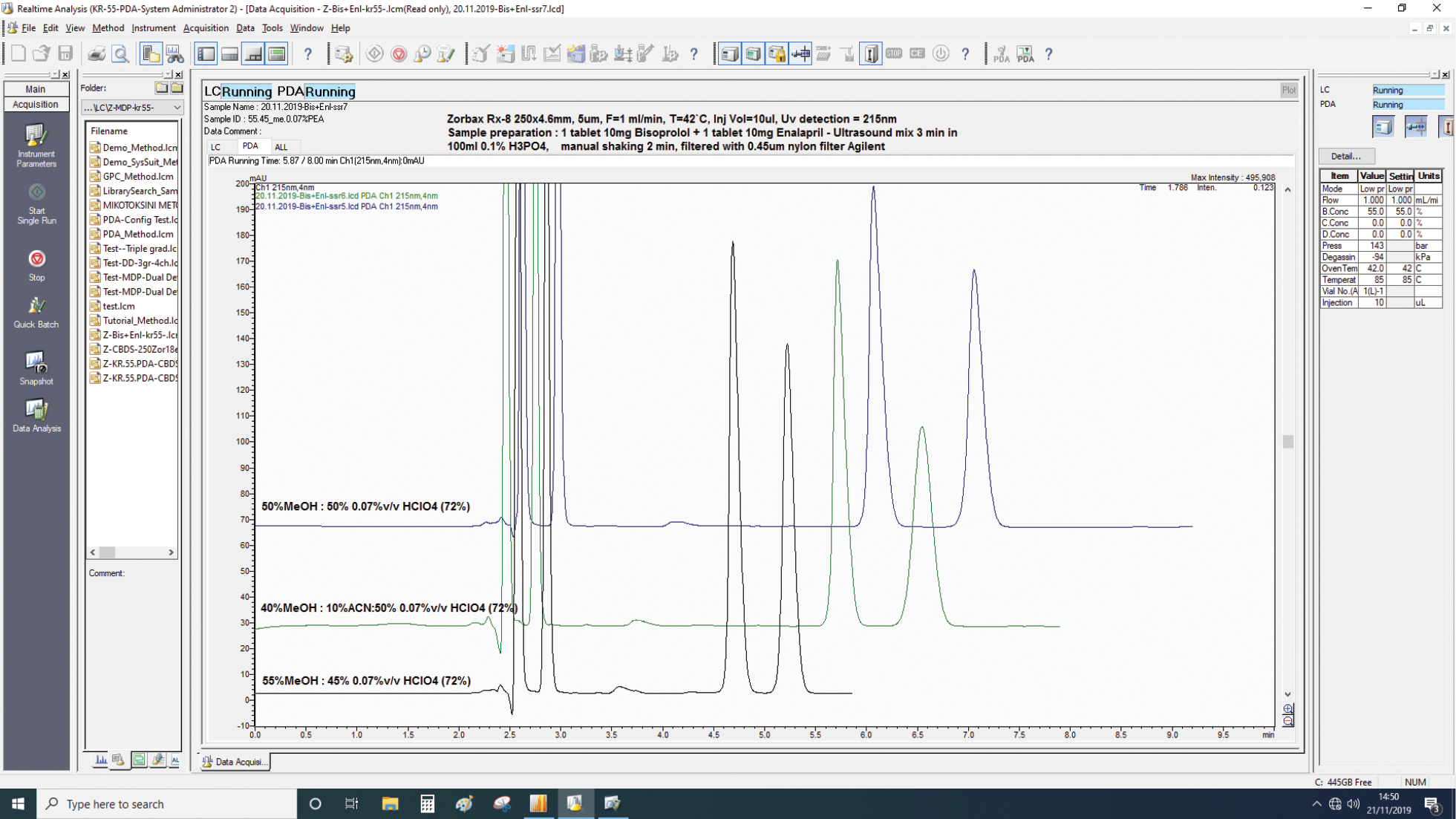
Elution profiles obtained for test samples prepared of bisoprolol+ enalapril tablets (10 + 10) mg using three different mobile phases: a) 55% metanol and 45% of 0.07% (V/V) perchloric acid; b) 40% metanol and 10% acetonitrile and 50% of 0.07% (V/V) perchloric acid; and c) 50% metanol and 50% of 0.07% (V/V) perchloric acid. First peak is bisoprolol and second enalapril. Chromatographic conditions: Shimadzu Nexera XR UPLC system, C-8 column Zorbax Rx (4.6 mm i.d. X 250 mm, 5 μm), flow rate 1.0 mL/min, column temperature 42 °C, injection volume 10 μL. |